Overview
The Health Professions Education (HPEd) Scientific Committee is the quality control for health professions education research, especially the Scholarship of Teaching and Learning (SoTL) research, conducted within the North-West University (NWU) and beyond. Experienced researchers and statisticians critically review proposals presented. The Committee provides researchers with meaningful feedback that helps to improve the quality and integrity of their research processes and procedures.
The HPEd Scientific Committee functions within the Centre for Health Professions Education (CHPE). This Committee enjoys support and collaboration from the Health Research Ethics Committee (HREC) and the Research Data Gatekeepers Committee (RDGC) of the North-West University. The Committee also receives technical and fiscal support from the Executive Dean of the Faculty of Health Sciences, the Deputy Dean for Research and Innovation, and the Deputy Dean for Teaching and Learning of the Faculty of Health Sciences.
Functions of the HPEd Scientific Committee
The Committee performs the following functions:
- Review degree and non-degree Health Professions Education research proposals from staff and students of the Faculty of Health Sciences, NWU and beyond.
- Provides balanced, meaningful and constructive feedback and guidance to researchers to improve their research proposals.
- The Scientific Committee reviews the proposals' scientific quality and recommends to the researchers regarding their HREC and/or RDGC applications.
- Provide training opportunities for their members.
- Consult with and guide academics in planning Scholarship of Teaching and Learning Research
Committee Members
|
Chairperson Prof Christmal Christmals Associate Professor Centre for Health Professions Education Faculty of Health Sciences North-West University |
Administrator Ms Paula Jardim Senior Administrative Assistant Centre for Health Professions Education Faculty of Health Sciences North-West University |
|
Prof. Jessica Pool Director, Centre for Health Professions Education Faculty of Health Sciences North-West University |
Prof James Avoka Asamani Team Lead, Health Workforce for the African Region Universal Health Life Cluster, World Health Organization, Africa Regional Office, Brazzaville, Republic of Congo Extraordinary Associate Professor, North-West University |
|
Dr Lucé Pretorius Subject Group Deputy Leader & Senior Lecturer Community Psychosocial Research (COMPRES) Faculty of Health Sciences |
Ms Sunshine Zenani Senior Lecturer School of Nursing Faculty of Health Sciences North-West University |
|
Dr Nokwanda E. Bam Senior Lecturer School of Nursing Faculty of Health Sciences North-West University |
Prof Petro Erasmus Associate Professor Psychosocial Health: Psychology Faculty of Health Sciences |
|
Prof Yolande Heymans Associate Professor Centre for Health Professions Education Faculty of Health Sciences North-West University |
Dr Susanne Jacobs Senior Lecturer Centre for Child and Youth Studies Faculty of Health Sciences North-West University |
|
Prof Anitia Lubbe Associate Professor Research Unit Self-Directed Learning Centre for Health Professions Education Faculty of Health Sciences North-West University |
Prof Juliet Nabyonga-Orem Health Systems Advisor World Health Organization, Namibia Country Office, Windhoek Namibia Extraordinary Associate Professor, Faculty of Health Sciences North-West University |
|
Dr Minnet du Preez Senior Lecturer School of Physiology, Nutrition and Consumer Sciences Faculty of Health Sciences North-West University |
Dr Belinda Scrooby Senior Lecturer School of Nursing Science Faculty of Health Sciences North-West University |
|
Prof Lizane Wilson Associate Professor Centre for Child, Youth and Family Studies Faculty of Health Sciences North-West University |
Dr Madeleine Uys Senior Lecturer Department of Pharmacology, School of Pharmacy Faculty of Health Sciences North-West University |
|
Dr Samantha Kahts-Kramer Senior Lecturer School of Human Movement Sciences Physical Activity, Sport and Recreation (PhASRec) Focus Area Faculty of Health Sciences North-West University |
Prof Leandi Lammertyn Associate Professor Department of Physiology Faculty of Health Sciences North-West University |
|
Dr Cornelia Schreck Senior Lecturer School for Human Movement Sciences, Recreation Programme Faculty of Health Sciences North-West University |
Dr Christiaan Bekker Senior Lecturer School of Psychosocial Health Faculty of Health Sciences North-West University |
Terms and Reference of Committee Members
- All members complete the NWU confidentiality agreement yearly.
- Scientific Committee meetings are compulsory. Any member not available for a specific meeting should inform the administrator, Paula Jardim, at least two days before the meeting.
- The administrator, in collaboration with the Chair of the Committee, will allocate a proposal submitted to reviewers (members).
- All reviewers must review the proposal using the HPEd Scientific Committee checklist (provided with each proposal allocation).
- Members are encouraged to consult with the Chairperson if they encounter difficulties reviewing the proposal.
- Members will have 14 working days to submit the review report. Reports for expedited reviews should be submitted within seven (7) working days.
- The reviewers will present their comments during the Scientific Committee meeting.
- The Committee shall decide on the proposal for approval and/or the level of corrections to be made.
- The Administrator must communicate the decision and collated comments to the researchers within two (2) working days.
- If the reviewers are satisfied with the rebuttal or the proposal at first submission, the Chairperson shall issue a letter of approval to the researchers. If the Chairperson is a researcher, the Deputy Chairperson will chair the meeting.
Figure 1 shows the processes a proposal submitted to the HPEd Scientific Committee goes through.
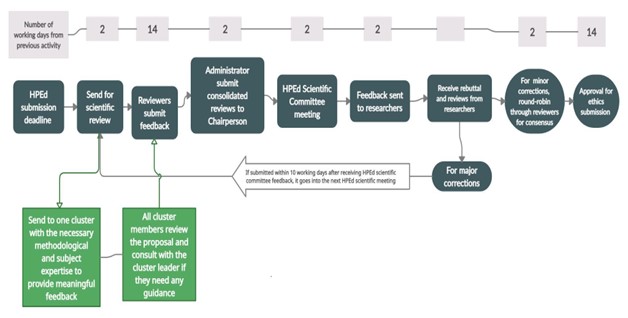
Application and Review Process
To submit your proposal to the HPEd for review:
- Consult with the Centre for Health Professions Education on the intended study (Optional) (appointments through Ms Paula Jardim - Paula.Jardim@nwu.ac.za)
- Access the applicable documents (processes, templates and forms) (Documents can be accessed here)
- Write the research proposal on the HPEd proposal template.
- Consult with a statistician if the study has a quantitative component.
- Submit the final proposal to the Scientific Committee with all the annexures and submission checklist to Ms. Paula Jardim [The nomenclature of the appendices is to allow for easy ethics application preparation].
- All similar documents (e.g., signed confidentiality agreement) of researchers should be combined into a single appendix file.
- Scientific Committee members review the proposal using the HPEd scientific review checklist (see a PDF copy to guide you).
- Researchers must attend the Scientific Committee meeting and present their study using the PowerPoint template provided.
- Feedback will be provided within two (2) working days after the Scientific Committee meeting.
- The rebuttal process is as indicated in the infographic in Figure 1.
- The Chairperson of the Scientific Committee issues an approval letter if the Committee has approved the proposal.

Figure 1
Table 1: Processes, templates and forms
|
Appendix |
Description |
Remarks |
|---|---|---|
|
Appendix A |
Cover letter to HPEd Scientific Committee |
Use template provided |
|
Appendix B |
Submission Checklist |
Use template attached |
|
Appendix C |
Executive summary |
Use template provided |
|
Appendix D |
SoTL Research proposal as approved by the small group panel |
Use the HPEd proposal template |
|
Appendix E |
Signed Code of Conduct for NWU Researchers |
Code of Conduct for NWU Researchers must be read and signed by all persons doing the research (gather and analyse the data). This will depend on the nature of your study. All signed codes of conduct must be combined into one (1) PDF and presented as one (1) Appendix. NWU Code of Conduct for Researchers can be downloaded from the NWU-HREC site at https://health-sciences.nwu.ac.za/healthethics/HREC/application-notification-forms |
|
Appendix F |
Signed NWU Confidentiality Agreements |
Everyone involved in the research must read and sign an NWU Confidentiality Agreement. All signed confidentiality agreements must be combined into one (1) PDF and presented as one (1) Appendix |
|
Appendix G |
2-page narrative CVs of researchers involved in the SoTL project |
A sample Narrative CV can be downloaded from the NWU-HREC site at https://health-sciences.nwu.ac.za/healthethics/HREC/application-notification-forms |
|
Appendix H |
Proof of ethics training of the research team |
Complete Modules: 1(Introduction to Research Ethics), 2.1 (Research Ethics Evaluation), 3.1(Informed consent) and South African Specific module. |
|
Appendix I |
Roles with responsibilities of research team members and research support team members |
Provide an outline explaining the role and responsibilities of each research team member. This includes members of the research team (PI & Co-I) and the research support team. |
|
Appendix? |
Informed consent form |
Should your research include more than 1 study population, e.g. students, academic staff/faculty management, etc., each population needs its own informed consent form as what/how/when/where, etc., may differ for each study population. The nature of your study will determine the number of appendixes here. The informed consent template can be downloaded from the NWU-HREC site at https://health-sciences.nwu.ac.za/healthethics/HREC/application-notification-forms |
|
Appendix? |
Informed consent checklist for NWU-HREC |
We strongly recommend using the NWU-HREC informed consent checklist for each of your informed consent forms to see if you have sufficiently addressed all the aspects and requirements. In the case of more than one informed consent form, each informed consent form must have its own checklist. The nature of your study will determine the number of appendixes here. The informed consent checklist can be downloaded from the NWU-HREC site at https://health-sciences.nwu.ac.za/healthethics/HREC/application-notification-forms |
|
|
Advertisements or recruitment materials |
Advertisements or recruitment materials you will use to approach your participants, e.g. announcements, information pamphlets, PowerPoint, etc., should be included and presented as a separate appendix. The nature of your study will determine the number of appendixes here. |
|
Appendix? |
Data gathering instrument(s) |
Each data-gathering instrument you will use in your research, e.g. survey / … interview schedule, etc., should be presented as a separate appendix. The nature of your study will determine the number of appendixes here. |
|
Appendix? |
Signed letter: Consultation with Statistical Consultation Services (only applicable if working quantitative) |
A qualified statistician should be consulted in the proposal development process. This ensures all quantitative assumptions, methods and calculations are aligned and appropriate. |
|
Appendix? |
Revised Ethics review checklist |
Use the template provided at https://health-sciences.nwu.ac.za/healthethics/hrec-applicant-tools |
|
If applicable |
Permission letters from governing bodies to conduct the research |
This will depend on the nature of your study.
|
|
|
Goodwill permission letters |
A letter seeking goodwill permission from institutions, individuals or groups of individuals involved in the study is required. |
|
|
Any other applicable documentation, e.g. MOU, contracts with collaborators/laboratories, permits, etc. |
|
|
|
Indemnity form |
Use the template provided at https://health-sciences.nwu.ac.za/healthethics/HREC/application-notification-forms |
|
|
Permission from the project leader if a study is done as an affiliated study under another study or a sub-study under a larger study |
Use the template provided at https://health-sciences.nwu.ac.za/healthethics/hrec-applicant-tools |
